This year, we’ve been looking into the cause of disease research. We’re trying to find outstanding organizations for donors interested giving to help out with research efforts to develop cures, or new treatments, to cancer and other diseases.
We figured that a logical place to start would be with two big-name organizations: the American Cancer Society and Susan G. Komen for the Cure. The first question we asked was “What do they do?”, and the first thing we found surprised us: funding research into cures or new treatments is a relatively small part of their activities.
American Cancer Society
The American Cancer Society’s 2008 IRS Form 990 demonstrates that ACS is not primarily a research organization.
The following chart shows a breakdown of the American Cancer Society’s Program expenses in 2008; explanatory notes follow. (Note these figures include the payments to affiliates — which themselves account for about 1/3 of the American Cancer Society’s expenses — according to the breakdown in Statement 7 of the 990.)
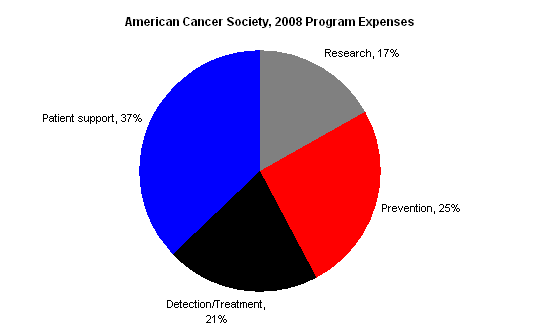
The 990 offers these notes to explain the different categories (quoted from Part III of the 2008 990):
- Patient support: Programs to assist cancer patients and their families and ease the burden of cancer for them.
- Prevention: Programs that provide the public and health professionals with information and education to prevent cancer occurrences or to reduce the risk of developing cancer. (My emphasis)
- Detection/treatment: Programs directed at finding cancer before clinically apparant & that provide information about cancer treatments for cure, recurrence, symptom management, and pain control.
- Research: Financial support provided to academic institutions and scientists to seek new knowledge about the causes, prevention. and cure of cancer, and to conduct epidemiological and behavioral studies.
Susan G. Komen for the Cure
Susan G. Komen’s 2008 audited financials paint a similar picture: it is not primarily a research (or research-funding) organization.
The chart below shows Komen’s 2008 program expenses, which include the central organization’s activities as well as those of the affiliates.
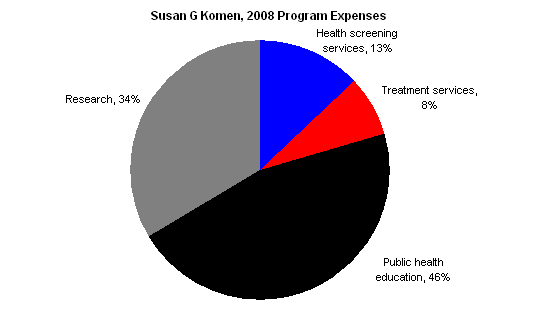
Komen’s 2008 Form 990, Schedule O offers additional explanation about the “public health education” category. The following is taken from the beginning of the section discussing its public health education program:
Komen has formed advisory councils to address the breast health and breast cancer needs of people from different cultures and backgrounds… We have developed a variety of educational materials for specific audiences in English and most are also available in Spanish… Examples of our education materials include – Breast Self-Awareness (BSA) cards in 12 languages for 14 specific audiences – General breast health and breast cancer brochures and fact sheets – Booklets with support information for survivors and co-survivors – Outreach resources including breast self-awareness information in CD-ROM, DVD or VHS formats.
Bottom line
We haven’t yet established anything about whether the American Cancer Society or Susan G. Komen is effective (or ineffective) at accomplishing their missions.
But we, at least, have been surprised by this fairly basic information.
- Both organizations seem to spend relatively small portions of their funds on researching new treatments or cures.
- Both organizations spend significant portions of their funds on “raising awareness” type activities.
I personally feel more negative on the two charities as a result of this basic check, because (a) I find research into cures/treatments as having a potentially huge upside in humanitarian terms, while public education and provision of existing treatments (in the developed world) don’t seem nearly so promising to me; (b) the “education” type activities are a red flag to me that research, specifically, may not have room for more funding.
Others may disagree, and I may change my mind after getting more information. But I wonder how many of the donors to these organizations have considered the variety of different activities that “fighting cancer” can mean, and considered whether they’d rather give to an organization that’s focused on a particular one of them.

Comments
I am somewhat surprised by your assessment of these organizations, although I am pleased that you have gone beyond the Program/Overhead ratio analysis that pervades most charity analysis.
There was an article in last week’s New Yorker about the incredible difficulty of developing new cancer drugs. I don’t think this is an argument against research, but it speaks to the extraordinary costs of developing treatments. Also, successful new cancer treatments are incredibly lucrative financially, so there is a greater amount of private capital dedicated to this type of research.
From my understanding, one of the greatest determinants of cancer survival rates is early detection. If this is true, than it makes sense to spend public and charitable dollars on public education campaigns. The more people who understand how to access health information and cancer screening services, the more lives can be saved by existing treatments.
Elie,
Thanks for the information.
Did you find anything specific on where the money they spend on research is going? If, say, Susan G. Komen spends 34% on research, how do they determine the recipients of that 34%?
Also, I’m not sure that their emphasis on educational activities suggests that research may not have room for more funding… I think you’re assuming that ACS and Susan G. Komen have carefully analyzed the research landscape and determined that there is only limited room for funding, leading them to devote more resources elsewhere. Although I’d be thrilled if that were the case, I doubt they’ve been quite that scientific about it when determining the breakdown of their activities.
Nick, our point is only that donors who specifically want to support the development of new cures or treatments should recognize that the two organizations above are not primarily focused on that activity.
My layman’s impression is that research into prevention (e.g. early detection) is underfunded and has more potential to save lives. Makes sense economically: you make more money curing already-sick people than keeping healthy people healthy.
Here is the Wired story that put this idea in me head.
Jason, we haven’t yet asked them how they decide which projects to support.
Seth, I don’t have the same intuition as you — I wouldn’t expect any systematic relationship between treatment vs. prevention and cost-effectiveness. Treatment has the advantage of providing resources solely to the people who need them.
Seth and Nick, in addition to the point raised by Elie, it’s not at all clear that early detection and screening are actually effective in saving lives from cancer. Detection and screening have indeed been associated with increases in five-year survival rates, but this may be just an illusion – early detection means that the five year “clock” starts counting when the cancer is at a less advanced stage, so people will appear to survive longer even if the course of the disease (including the likelihood of eventually dying from it) hasn’t changed at all. The fact that age-adjusted cancer mortality (e.g. the fraction of the population of a certain age that dies of cancer each year) does not appear to have dropped much is very consistent with this interpretation.
There was a good post on this at Marginal Revolution last year, which addresses the Wired article that Seth brought up.
Given this background, I find ACS and SGK’s significant focus on prevention/detection to be especially unfortunate. Given that (a) there is a natural and quite human impulse to believe that if we just act responsibly and prudently we can ensure good health, (b) people have a very strong intuition that “an ounce of prevention is worth a pound of cure”, and (c) the data appear at first glance to suggest that prevention is effective, I can see why a lot of people might reasonably feel that money for detection is a good idea. But sadly, I think that in all probability it is likely a waste of money, at least at present (things could change in the future).
Excellent points, Dario.
Yes, existing screening tests are only useful in a few very specific cases. Pap smears, colonoscopies, and mammograms, and even those only in certain populations. Many other screening tests have been tried, but most others have not been beneficial (too many false positives leading to removal of benign tumors, etc.) Remember the controversy last year regarding mammograms for women in their 40’s? Expanding screening tests beyond these populations would be actively harmful.
Also, I’m not sure how useful raising awareness of cancer would be…a person only needs to see their doctor to be told which tests to get (if any) and when.
There are only a handful of behavioral changes that really make a difference in preventing cancer (smoking, IV drug use leading to hepatitis, sunscreen) but overall, there’s not much you can do to prevent it, in stark contrast to heart disease.
One last thing…not sure why people think a preventative medication/test would not be lucrative for the inventor…statins and Plavix are basically preventative measures, and they’re some of the most lucrative drugs.
I think a lot of cancer are preventable and are linked to products that are being pushed by various industries that don’t pay the real cost of their activities: it is true for tobacco, alcool and probably many other industrial foods and drinks as well as chemicals in our environment. Considering the number of people impacted and prematurely killed by tobacco and the proportion of cancers that are tobacco related it seems to make sense to push for better regulation and maximal prevention: California with a smoking prevalence around 12% is a key example. How about assessing the spending by comparing the amounts allocated to the number of patients/victims involved?
In any case, putting a premium on research does not appear to be the most effective way to deal with cancers that can be prevented as progress -unfortunately- has been very limited in terms of cure. Not a very optimistic thought but a realistic one?
I can’t speak for Komen, but I did work for ACS about a decade ago for a period of 3 years. I’m not invovled with them now, and don’t consider myself a loyalist by any stretch.
That said, I think you need to look not just at the 2 more prominant cancer nonprofits, but also at the broader area of cancer research. The National Cancer Institute has over $4.8 Billion at their disposal for cancer research, which is over six and a half times ACS’s entire budget… for everything.
With it’s limited funds, ACS chose a strategy that leverages it’s strengths. The limited research budget goes to small, promising and/or innovative projects that generally don’t get funded by the federal government, filling a much needed niche. Your comments notwithstanding, early detection and prevention and advocacy work goes a long way to reducing the incidence and survivability of cancer. In NYC, ACS was at the front of the parade to get smoke free workplace (including bars and restaurants)legislation enacted. Edcuation that encourages men and women to get colonoscopies works for one of the more preventable and treatable cancers if caught early, and deadly if caught late.
pI agree with an earlier post that lauds you for going beyond income and expense, but as you say a lot more research needs to be done.
As a charity that has been awarded 4 stars by Charity Navigator, it is our belief that funds spent on education are every bit as important as the money invested in research. What good are therapeutics if patients are poorly educated about about their disease and how to deal with it? If physicians are not proactively engaged, how will the next generation of researchers be encouraged to conduct experiments aimed at specific cancers? As for awareness, at the time of diagnosis, most patients know very little about how best to deal with their cancer. Programs that address this are valuable, too.
Thanks for posting this article. It was really interesting to see the breakdown to this level for such a major brand organization. Although the public education component was vastly larger a percentage than I expected, I tend to believe Komen for the Cure is operating very close to optimal and in line with stated mission, goals and objectives.
I work for the American Cancer Society and people I meet are often surprised at how broad our mission is and how much we actually accomplish. We have learned so much about how to prevent and detect cancer in the more than six decades we’ve been funding cancer research. As Bill rightly points out, the American Cancer Society has used that knowledge to change societal norms, promote proven cancer screenings and encourage healthy lifestyles to save lives from cancer. In fact, more than 50 percent of cancer deaths could be avoided if we did all of the things we know to reduce cancer risk. We have seen these efforts pay off in the continued declines of cancer incidence and mortality rates since the early 1990s.
That said, it’s important for your readers to know that the American Cancer Society continues its strong commitment to cancer research — we are the largest private funder of cancer research, second only to the federal government. We are also proud to say that we are as tough on ourselves as we are on the disease we fight, holding ourselves accountable to aggressive goals. We make our Strategic Plan and progress report available to the public on our Web site every year which you can find here http://tiny.cc/1e2e0.
Anyone interested in this topic and how Komen approaches “the cure” should read the book “Pink Ribbons, Inc.” for a fascinating look into the race and gender politics of breast cancer prevention organizations.
I agree that just putting public awareness out there doesn’t necessarily help people who already have the disease. The biggest issue I have with the current take on awareness (which usually means advocating for people to get tested) is that it doesn’t address the environmental causes of cancer. It’s not just lifestyle choices that matter, it’s also chemicals and plastics that we are in contact with everyday.
Sometimes it is the same corporations that are promoting their products with the pink ribbon (for example Ford’s Warriors in Pink campaign) that are also producing the pollution that can cause health problems.
Just a couple more things to add to the great discussion…
Just wanted to voice a bit of skepticism about many of the views expressed above:
First, there is serious doubt about whether chemicals in the environment, with the exception of asbestos (which really is very carcinogenic), are a major cause of cancer. Here, for example, is a skeptical point of view (with which I don’t necessarily fully agree).
Second, while smoking really is the single largest cause of cancer (a third of all cancers is the number I’ve seen), it’s not at all clear that secondhand smoke is a major cause of cancer. The data are questionable, and anecdotally I’ve gotten the impression that the public health community trumps up the risks of secondhand smoke as a (well-intentioned) attempt to make people feel more guilty about smoking and thus smoke less. So the value of ACS’s efforts to ban smoking in the workplace are not above reproach.
Third, public education campaigns are only as good as the message they send. If screening/detection is of limited value, then campaigns to promote it will of course also be of limited value. Raising awareness of a disease could in theory be useful, but there’s a zero-sum component to it – public attention is a finite resource and drawing attention to one disease can just reduce attention to others (which may be no less important). Finally, public campaigns to reduce smoking can save lives if they are effective, but smoking is addictive and behaviors are hard to change – it’s not clear to me just how cost-effective such efforts are (data on this would be very useful).
Like Elie I have the intuition that research is the most potentially promising avenue, and the low percentages devoted to research by these organizations suggests that they may not be finding much worthwhile, unfunded research.
Thanks for the comments. We’re planning to continue our research into and discussion of disease research organizations in future posts.
Elie,
Many organizations allocate a portion of their special event costs to education and awareness for the cause, and those expenses enlarge the educational component of their organizational pie chart as a result. Organizations that are particularly dependent on special event fundraising and that allocate educational event costs in this way may show higher educational costs overall.
You might check out the Tinkelman case study on the Pallotta TeamWorks website which looked in particular at the way Avon was allocating costs on its events. Full disclosure: Dan Tinkelman was expert counsel for Pallotta TeamWorks in litigation against Avon.
Dan, thanks for the comment.
I briefly looked at the Tinkleman case study (PDF) you mentioned, and it seems the main point is that organizations (including Komen, which is mentioned specifically) have a strong incentive to minimize reported fundraising costs (due to the prominence of the overhead metric as a standard for evaluating charities).
Are you saying that it’s more appropriate to consider the education/awareness activities as fundraising for the organization itself rather than a distinct program activity?
Based on the description Komen provides of its “education” activities, I wouldn’t guess that events, specifically, account for the majority of that category’s expenses.
In addition, page 2 of the Tinkleman case states, “Komen does not run the events itself. Its financial statements report only the net proceeds it receives, without reporting any of the related event expenses.” That is, event costs are sometimes not reported at all as they’re “run” by other organizations.
Hi Ellie,
Well, as you know, I think the whole system is broken. I think it’s fine – actually proper – for certain expenses associated with events to be categorized as mission expenses, so long as the fact that it’s being done that way is made transparent to the donor. In other words, I don’t think it’s cool to tell a donor 80% goes to the cause when the donor things “the cause” is, for example, diabetes research, when in fact what the organization means is research and education, and only 50% is really going to research.
I believe Komen now runs their events on their own, but that may just be starting – so you are probably correct that there were no event-related educational expenses showing up in their educational expenses.
This is Cheryl Jernigan, and I’m an Advocate in Science for Susan G. Komen for the Cure, that is, I am a breast cancer community advocate who helps guide Komen for the Cure’s research program. Komen has operated for nearly 30 years on the promise to discover and deliver the cures for breast cancer. Such a commitment has to be multi-faceted — touching every area of this disease, from research to education to treatment and support for the people who are living with the disease today. The $500 million we’ve invested in research so far has led to advances in early detection, screening, treatments like tamoxifen and to less invasive treatments like lumpectomies rather than mastectomies for many women. Now, Komen is focused on the research that has the best chance of translating into clinical practice and cures within 10 years. Research is a critical component of our mission and always has been. But research alone won’t save lives. People need to be able to access the research, and they need knowledge, so Komen has invested another $900 million into programs to help people get educated, get access to screening and treatment if they can’t afford it and get help through treatment and beyond. It helps, if you’re considering which programs to support, to look at what an organization invests in its total mission. All told, 84 cents of every dollar Komen raises gets re-invested into that total mission to discover – and deliver – the cures.
Hi Cherly,
Thanks for commenting. I’m particularly interested in something you wrote:
We’re very interested in Komen’s assessment of its track record of success for funding research. Can you point us to more information about the role Komen played in the development of tamoxifen and the impact tamoxifen has had on breast cancer? What advances in early detection has Komen caused and to what degree have these prevented suffering from cancer?
More broadly, do you have a more comprehensive assessment of the impact of the research you’ve funded? For example, I’d be interested to know if, say, you’ve done a citation analysis of the research you’ve funded. That is, are papers published due to Komen funding heavily cited by other scientists? What other measures do you use to evaluate the effectiveness of your research funding and how does Komen perform on those measures?
I am a 5 time cancer survivor.I have never had the “PINK” kind (Breast) I wouldn’t be here if it wasn’t for early detection & research.
I find it offensive that so many companies jump on the pink band wagon. Researching only 1 type of cancer?! Really? There are more than 200 different types of cancer. Who is researching those? Not KOMEN!
The American Cancer Society has been around for almost 100 years. They are the #1 funder for cancer research next to the Government. They have had a hand in every major cancer break through. The PAP, PSA Screening, Mamogram, all the differnt drugs being used. They have funded 44 Dr.s that have won Nobel Prizes.
The ACS holds a non for profit tax status. Komen doesn’t.
Personally, I want all cancer to be researched, not just breast cancer.
“All told, 84 cents of every dollar Komen raises gets re-invested into that total mission to discover – and deliver – the cures.”
What does “”re-invested” mean, Cheryl Jernigan? What are the expenses? Investments have expenses.
I am very skeptical about any of these fund raisers. There is no real financial statements easily accessible showing the bottom line: How much money NET and how that is distributed. Seems to me the companies producing TV commercials and printing T-Shirts are making money. Everyone wants to run around and say how far they walked to raise money for cancer, but how many of those people actually directly help a person with cancer? Wouldn’t that time and money be better spent giving it directly to a person that has the disease? We live in a society that is great about promoting information, everyone refers you to some organization that has information. But what about getting actual help from a live person who does the cooking and the ass wiping for the dying patient? Seems the “work” is left to the laborers at the bottom of the medical industry while the rich “go for a walk” to shed a few pounds in the name of “charitable awareness efforts” and do “fund raisers” that serve meals and drinks full of cancer causing chemicals. When my wife was dying of brain cancer, (and my son who has Autism) needed my help, I didn’t run off and do any fund raisers because I had no time or money to do that sort of pointless exibition, I had to roll up my sleeves and take care of them. Instead of walking around in a new pink ribbon T-Shirt, those people should walk over to a hospital and lend a hand and write a check directly for the patient’s medical bills!
Do the AMC and Susan B Komen work together? If I like one but not the other, will my donations go to both?
Regarding Cancer Society, what is the percentage of total outlay that goes to actual charity compared to administrations and fund raising activity.
Of all the money that was spent on research, has any of the money produced results, after so many many years. What are they, if any.
If any of the drugs invented has been funded by Cancer society, why is it still so very expensive.
Thanks
John Leung
I read this report for a class speech and I do not think the ACS is doing a bad job on their funding. My uncle is currently dying of cancer that went undetected by his doctor for a year until it had matastasize to other parts of his body. Research is a important part of understanding the disease but money is also needed to teach doctors and patients the warning signs, and the prevention funding helps to acknoledge what is needed to be done to prevent such cancers like lung cancer,mouth cancer ect. Patient support helps the patient understand they are not alone and sometimes that thought is all they have to hold on to. So, even though the funding doesn’t seem well spread out or focusing on important parts like research each part to a cancer patient and their family is important.
If you look more into the American Cancer Society you will find they funded the researchers of the PapSmear, Glevac, Tomaxifin (sp), and CemoTherapy as well as the CatScan. You will also find that only about 3% goes into managment and facility costs with over 80% going directly to help patients, research and awarness. Please do more research… http://www.cancer.org before being negative
Of COURSE the ACS and Cancer.org will SAY they spend money on research! Bottom line, they don’t. Take breast cancer, they say mammograms “prevent” BC due to early detection. Most tumors turn out to be false positives. Annual exams increase the risk by 2% per year, over 10 years that’s 20% increased risk. Here’s the math: a $150 mammogram for 70 million American women over 40 amounts to $10 BILLION per year for the industry. Can you imagine this kind of money disappearing from that industry? Do you really think they would let it! They use a cancer causing machine, and before you die, you get to spend all your money on “treatments” for cancer CAUSED by that machine, that will leave you bankrupt first, dead next. Pretty profitable, I’d say!
hmmmm, the way I read the tax return, they spent $390,305,361 on salaries, pensions, benefits and payroll taxes while spending a mere $4,406,038 on research. They spent more than 4 TIMES the research amount attending conferences ($18,158,259). The ACS Action Network is the “advocacy arm” for big pharma and also own millions of dollars worth of tax exempt real estate. They lobby for Robert Wood Johnson Foundation. The RWJF was created by the founder of Johnson and Johnson. As of their 2009 annual report, RWJF owned 42,343,491 shares of JnJ stock. RWJF has give half a billion dollars in “grants” for tobacco-control/smoking bans. So, who do you think has the market cornered on nicotine REPLACEMENT therapy products? JOHNSON & JOHNSON. The American Cancer Society, American Heart Association and American Lung Association split a $99,000,000 tobacco control grant. According to RWJF, THEY CREATED and FUNDED Tobacco Free Kids. Their start-up grant was $84,000,000. So you see, all these organizations got money from the “non-profit” of J&J that owns J&J stock who profits from big pHARMa’s form of nicotine. And our nation’s business owners are losing their livlihoods to this fraud with smoking bans for the profit of pharma!
If you follow the dictates that are passed down through various organizations TO various organizations, you will find that the funding comes from places like the Robert Wood Johnson Foundation in the form of grants. That is the Johnson and Johnson Empire of 250 pharmaceutical companies. If they had a cure, they would loose money. However, to provide ‘counseling’ of how to take your medicines and advise for specific tests, it puts money into their pockets. … The RWJF is Johnson and Johnsons foundation, JnJ owning at 250 pharmaceutical companies. They set up ‘Tobacco Free Kids’ with at $84 million as their lobby group. The Johnson and Johnson Empire makes the no smoke products. …. Follow them for the ‘obese’ and other issues. … Merchandising is now population control!
How many days must my comments be under moderation? I can back up everything I say.
Cheryl, your comment seems like doublespeak to me.
“Research is a critical component of our mission and always has been. But research alone won’t save lives. People need to be able to access the research, and they need knowledge”? What does that mean? I think most people believe that “research” means “research trying to find a cure for breast cancer.” I assume that if you manage to find a cure for cancer, everyone will know about it and there will be no need for an education campaign.
I agree that people would need help accessing such a cure (health care costs are enormous), but you haven’t found it yet, so you’re putting the cart a bit before the horse!
As for making screening available to low-income people, I take your committment to such things with a grain of salt, given that you just cut your funding for cancer-related screening from Planned Parenthood.
If you’re talking about awareness of cancer generally (what “research” is involved there, I don’t know), I think everyone’s already aware of cancer.
I felt that it was important to list the research accomplishments of the American Cancer Society. We fund young cancer researchers and 46 ACS funded scientists have been awarded with the Nobel Prize for their cancer research. Please take time to review the research facts listed below.
We Save Lives
Timeline of Research Accomplishments
1946 ACS Research Program begins with $1 million raised by Mary Lasker.
1946 First ACS supported researchers, Wendell M. Stanley, PhD, in Chemistry and
Hermann J. Muller, PhD in Physiology or Medicine win Nobel Prizes for crystallizing
a virus and discovering mutations due to Xrays, respectively.
1946 ACS began a Crusade to gain acceptance of the Pap Test, developed by George
Papanicolaou, MD, PhD in 1941. The widespread adoption of this simple test has
resulted in more than a 70% decrease in mortality from cancer of the uterine cervix
Papanicolaou was funded by ACS in the 50’s and early 60’s for other cancer related
research.
1947 ACS funded Sidney Farber, MD, obtains remission in childhood leukemia with antifolate
drug, aminopterin, the first successful chemotherapy of cancer, setting the groundwork for the first human clinical trial.
1953 ACS funded James Watson, PhD, (with Francis Crick, MD) establishes the double helical structure of DNA for which they were awarded the Nobel Prize in 1962.
1954 ACS Hammond and Horn study in the U.S., and British Doctors’ Study in the U.K. confirm the link between smoking and lung cancer.
1955 ACS funded Charles Huggins, PhD, pioneers early work showing that both prostate and breast cancer were related to sex hormones. He received the Nobel Prize in 1966.
1955 ACS funded Emil J. Freireich, MD, and colleagues design first scientific clinical trial for combination cancer chemotherapy; by
1962 achieve a 15% cure rate in childhood leukemia.
1958 ACS postdoctoral fellow Matthew Meselson, PhD, and Research Professor Franklin Stahl, PhD, demonstrate how DNA replicates, by conservation of one strand of the double helix in each of the two daughter strands.
1958 5-fluorouracil, chemotherapeutic drug used in many cancers, is synthesized by Charles Heidelberger, PhD.
1959 Beginning of ACS Cancer Prevention Study I (CPS I), which shows cigarette smoking was responsible for early death from lung cancer.
1959 Stanley Cohen, PhD, discovers growth factors; wins Nobel Prize in 1986.
1962 Discovery of restriction enzymes by Hamilton Smith, MD, and Daniel Nathans, MD, crucial to the later development of genetic engineering and gene cloning. Smith and Nathans won the Nobel Prize in 1978.
1964 Due to the findings of ACS CPS I, the Surgeon General concurs that cigarette smoking is irrefutably linked to cancer.
1965 Former grantee Robert Burns Woodward, PhD, receives the award in Chemistry for his achievements in determining how the body uses small compounds to build organic molecules for life’s functions.
1966 Elwood Jensen, MD, and Eugene de Sombre, PhD, describe the existence of protein receptors that bind to sex hormones and carry out their functions.
1966 Henry Lynch, MD, describes the first hereditary cancer family syndrome.
1968 Donald Pinkel, MD, uses high dose radiation to prevent central nervous system relapses and achieves 35% cure in childhood leukemia.
1969 Former grantee Max Delbruck, PhD, shares the Nobel Prize in Physiology or Medicine with Drs. Salvador Luria, MD, whom we also funded, and Dr. Alfred D. Hershey. Together they discovered how DNA replicates itself and the genetic structure of viruses.
1970 The first cancer causing gene, or oncogene, is identified as the src (sarcoma) in a chicken tumor virus by grantees Peter Vogt, MD, and Hidesaburo Hanafusa, PhD.
1970’s ACS allots $3 million for the development and testing of the first biological therapy, alpha interferon, now used in the treatment of some forms of leukemia and childhood Wilm’s tumor of the kidney.
1970’s Epidemiological evidence analyzed by Brian McMahon, MD, shows that breast cancer was related to length of a woman’s lifetime exposure to reproductive hormones.
1970’s ACS invests over $1 million to demonstrate that mammography was the best tool to use to detect breast cancer early in the BCDDP study.
1970’s ACS Research Professors Joseph Bertino, MD, and Robert Schimke, MD, work out mechanisms of drug resistance.
1971 Leading role in the passage of the National Cancer Act which is considered the most dramatic piece of health legislation ever enacted.
1971 Grantee Irving Selikoff, MD, demonstrates that occupational exposure to asbestos increases risk of rare lung cancer, mesothelioma.
1972 First grant in anti-angiogenesis goes to Judah Folkman, MD.
1972 ACS funded E. Donnall Thomas, MD, pioneers the technique of bone marrow transplant to treat cancer. He received the Nobel Prize in 1990.
1972 Phillip Sharp, PhD, William Sugden, PhD and Joseph Sambrook, develop the technique of using agarose gels and ethidium bromide staining for detection restriction-enzyme fragments.
1973 ACS grantee Stanley N. Cohen, MD, creates “recombinant DNA.”
1973 ACS funded Paul Berg, PhD, clones the first gene Nobel Prize in 1980.
1974 — ACS funded V. Craig Jordan, PhD, shows that tamoxifen could prevent breast cancer
in rats by binding to the estrogen receptor.
1974 ACS grantee Lawrence Loeb, MD, PhD, proposes that random mutations must accumulate much faster than is normal inside cells that become malignant, perhaps due to malfunction in DNA replication or repair.
1975 Field of psycho-oncology is established by Jimmie Holland, MD.
1976 ACS funded J. Michael Bishop, MD, and Harold Varmus, MD, discover oncogenes in normal DNA suggesting that a normal gene already present in the cell has the potential of becoming an oncogene. They were awarded a Nobel Prize in 1989.
1976 ACS Professor of Clinical Oncology Lawrence Einhorn, MD, develops combination
chemotherapy protocal to treat metastatic testicular cancer; results in 50% cure rate.
1977 The Great American Smokeout began.
1978 ACS Research Professor Tony Hunter, PhD, and grantee Bart Sefton, PhD, provide the first clue to the biological function of an oncogene, in this case an enzyme involved in cellular communication.
1978 ACS funded Clara Bloomfield, MD, demonstrates chromosome rearrangement in leukemia and opened up the field of cytogenetics.
1978 Tamoxifen is approved by the FDA for treating estrogen receptor positive breast cancer. ACS funded Bernard Fisher, MD, Richard Love, MD, and V. Craig Jordan, PhD, develop and carry out the first trial of tamoxifen to prevent recurrence in breast cancer survivors.
1978 ACS funded Walter Gilbert, MD, (and Frederick Sanger, PhD) develop technique to sequence DNA. They received the Nobel Prize for this in 1980.
1979 ACS Research Professor Robert Weinberg, PhD, demonstrates thefirst biologically active human oncogene from a human bladder cancer; over 50 human oncogenes are known today.
1979 ACS funded Arnold Levine, MD discovers the p53 protein, later shown to be a tumor suppresser gene mutated in over half of all cancers.
1979 A team led by Harold Varmus, MD, shows that a family of enzymes known as protein-tyrosine kinases are involved in the regulation of cell growth.
1980 Early detection guidelines are set for breast cancer.
1980 Grantee Alfred Gilman, MD, PhD, discovers G-proteins and begins to elucidate their role in signal transduction, for which he wins a Nobel prize in 1994.
1981 ACS grantees T. Ming Chu, PhD and Gerald P. Murphy, MD, DSc, develop the prostate specific antigen (PSA) test for screening and early detection of prostate cancer.
1981 ACS Research Professor Robert Weinberg, PhD, isolates neu oncogene from rats — later shown to be homolog of human Her2/neu oncogene (breast cancer.)
1981 Curt I Civin, MD, discovers the CD34+ protein on the surface of blood stem cells that identifies those among other blood cells in bone marrow; receives four US patents to harvest stem cells without puncturing bone.
1982 ACS begins Cancer Prevention Study II (CPS II), a study of 1.2 million men and women to analyze risk and preventive factors involved in cancer.
1982 ACS grantee Richard Palmiter, PhD, produces the first “transgenic mouse”–a mouse with a gene for rat growth hormone.
1982 ACS Clinical Research Professor Ronald Levy, MD, successfully treats a lymphoma patient with a monoclonal antibody.
1983 Alfred G. Gilman, PhD, describes the role of the “G-protein” in transduction of growth factor signals from the cell surface to turning on of genes in the nucleus. Receives Nobel Prize for this in 1994.
1983 Ralph Steinman, MD, discovers the role of dendritic cells, which later become the basis of therapeutic cancer vaccines.
1984 ACS grantee Sherie Morrison, PhD, makes a chimeric monoclonal antibody that is half mouse, half human, and can thus be produced in large quantites by mice but not be rejected by humans.
1984 Grantee Robert Sinsheimer, PhD, proposes the first collaborative project to sequence the entire human genome.
1985 ACS Research Professor Edward Harlow, PhD, clones the mutant p53 gene.
1985 ACS funded Bernard Fisher, MD, demonstrates that lumpectomy plus radiation is equivalent to mastectomy for breast cancer survival.
1986 ACS funded Robert Weinberg, PhD, clones the first of some 20 now-known tumor-suppressor genes; the retinoblastoma gene of a childhood eye cancer.
1986 Former grantee Stanley Cohen, Ph.D. shares the Nobel Prize in Physiology or Medicine with Dr. Rita Levi-Montalcini, honored “for their discoveries of growth factors.”
1986 ACS grantee David I. G. Kingston, PhD, develops Taxol (paclitaxel) and Taxotere (docetaxel) as useful cancer drugs.
1987 Grantee Mario Capecchi, PhD, suceeds in “knocking-out” specific genes in mice, thus allowing a study of the function of a gene of interest in an intact animal.
1987 ACS Research Professor David Baltimore, PhD, and ACS grantee Owen Witte, PhD, show that the abnormal fusion protein characteristic of chronic myelogenous leukemia is a tyrosine kinase enzyme.
1988 Former ACS postdoctoral fellow Philip Leder, PhD, obtains patent for the “Harvard Mouse,” a genetically engineered transgenic mouse with a human cancer-causing gene.
1988 ACS funded Dennis Slamon, MD, discovers that the her2/neu human epidermal growth factor receptor 2 is overexpressed in 15-30% of breast cancers, and is an unfavorable prognostic feature.
1990 Walt Disney – ACS Research Professor Mary-Claire King, PhD, localized the BRCA1 gene for inherited susceptibility to breast cancer to a specific site on chromosome 17.
1990 ACS Clinical Research Professor Waun Ki Hong, MD, completed the first chemoprevention trial to show efficacy of a vitamin A analogue against mouth and throat tumors.
1990 CPS II data show decreased risk of fatal colon cancer associated with a diet rich in fruits
and vegetables (Michael Thun, MD.)
1991 ACS funded researcher, Paul Fischer, MD, shows that young children recognize Joe Camel as easily as Mickey Mouse, demonstrating that the cartoon character reaches an audience well under the legal smoking age.
1991 Data from CPS II demonstrate a decreased risk of colon cancer in people who take aspirin regularly (Michael Thun, MD.)
1991-93 ACS Research Professor Bert Vogelstein, MD, and Richard Kolodner, PhD, clone
several genes for inherited susceptibility to colon cancer.
1992 The Mammography Quality Standards Act of 1993 passed; smoking drops from 45% of the population in 1946 to 25%.
1993 Grantee Victor Ambros, PhD, discovers the first RNAi (RNA interference) in a small roundworm; ten years later this phenomenon is exploited to locate genes important in obesity and cancer.
1993 Drop in smoking reported from 45% of the adult population in 1946 to 25%.
1994 Exercise reduces risk of breast cancer in premenopausal women Brian Henderson,MD.
1994 Taxol (paclitaxel) is approved as second line therapy for advanced breast cancer, based on the work of ACS grantee David G. I. Kingston, PhD. Dr. Kingston’s research also leads to the FDA approval of Taxotere (docetaxel) for breast cancer in 1996.
1994 A genetic screening test for a rare form of thyroid cancer is developed by Ruth Decker, MD. Affected children who have their thyroid glands surgically removed before age two do not develop cancer.
1995 Data from CPS II indicate a decreased risk of colon cancer in postmenopausal women on estrogen replacement therapy (Eugenia Calle, PhD).
1995 Grantee James Darnell, MD, describes the JAK-STAT pathway through which signals from outside a cell are transmitted to the cell nucleus to activate genes.
1995 ACS grantee Walter Willett, MD, DrPH, shows that a Mediterranean diet rich in tomato sauce and olive oil helps prevent prostate cancer.
1995 Former grantee William Catalona, MD, is the first to show that the prostate specific antigen (PSA) test can be used to screen for prostate cancer in asymptomatic men.
1995 ACS launches Behavioral Research Center directed by Frank Baker, PhD.
1996 ACS Guidelines on Diet, Nutrition and Cancer affirm that one third of all cancer deaths can be prevented through healthy eating and physical activity.
1996 CPS II shows increase in lung cancer deaths among nonsmoking spouses of smokers.
1996 CPS II data shows secondhand smoke increases mortality from lung cancer and heart disease.
1996 ACS Clinical Research Professor Waun Ki Hong, MD, initiates phase I trial of p53 gene therapy in lung cancer.
1996 Camptosar (irinotecan) is approved for recurrence of metastatic colon cancer, based on earlier work of ACS grantee MilanPotmesil, PhD, who developed drugs that interfered with DNA-unwinding enzyme, topoisomerase.
1996 Larry Clark, MPH, PhD, reports that selenium reduces risk of lung, colon, and prostate cancer.
1997 ACS Research Professors Thomas Cech, PhD, and Robert Weinberg, PhD,
independently clone the gene for telomerase, an enzyme important in cancer cells.
1997 ACS dedicates the majority of its research dollars to beginning investigators.
1997 Former ACS grantees Judah Folkman, MD, and Timothy Browder, MD, cure cancer in mice by blocking the blood supply of tumors with angiostatin and endostatin.
1997 First overall downturn in cancer mortality is documented: overall cancer death rates fell 0.5% per year between 1991-1995.
1997 FDA approves monoclonal antibody therapy (rituximab, Rituxan) for B-cell lymphoma, based on work of ACS Clinical Research Professor Ronald Levy, MD.
1998 Overall five year survival rates are improved to 58%.
1998 First overall decrease in new cases of cancer; overall cancer incidence rates fell 0.7% per year between 1991-1995.
1998 Bernard Fisher, MD, reports that tamoxifen reduces the incidence of breast cancer by 45% in high risk women.
1998 ACS targets 10% of its research expenditures to special areas of prostate cancer research.
1998 DNA Microarray, or the Gene Chip, developed by Patrick Brown, MD, PhD, becomes usuable for human gene analysis with the “cluster analysis” method of Brown and former grantee David Botstein, PhD.
1998 Grantee Craig Mello, PhD, shows that small double-stranded RNA (siRNA) can interfere with specific gene expression, opening up a new field for possible drug development.
1998 Former grantee Dennis Slamon, MD, shows that a genetically engineered, “humanized” monoclonal antibody, Herceptin (trastuzumab) improves survival of women with advanced breast cancer. FDA approval granted.
1998 Former grantee Craig Mello, PhD, discoveres RNA interference (RNAi) inhibits gene expression. This technique is hailed by former grantee and Nobel Laureate Phillip Sharp, PhD, as a “gift from heaven.”
1999 Former grantee V. Craig Jordan, PhD, reports that raloxifene reduces the risk of breast cancer by 76% in postmenopausal women with osteoporosis.
1999 The ACS’ targeted research area changes to “Cancer in the Poor and Underserved.”
1999 ACS Research Professor Robert A Weinberg, PhD, annouces that he has been able to turn a normal human cell into a cancer cell with three defined genetic elements: an oncogene, a gene for a product that inactivates two suppressor genes, and the gene for telomerase.
2000 Brian Druker, MD, begins phase II trial of STI-571, a drug that inhibits an enzyme present only in chronic myelogenous leukemia cells, after it induced remission in all 31 CML patients of his phase I study.
2000 Draft sequence of the human genome announced in June.
2000 A team of scientists that includes ACS Clinical Research Professor Waun Ki Hong, MD, announces that the combination of chemotherapy with p53 gene therapy caused tumors to shrink in 25% to 30% of head and neck cancer patients.
2000 The DNA Microarray Chip technology is successfully used by a team of 30 researchers, including ACS Clinical Research Professor Ronald Levy, MD, , to identify two types of lymphoma that look the same under the microscope, but that respond very differently to standard therapy.
2000 John Mendelsohn, MD, reports that a monoclonal antibody IMCC225, against the epidermal growth factor receptor, developed while he was an ACS Professor of Clinical Oncology, is effective in the treatment of refractory colon and head and neck cancers.
2000 Mylotarg, a chemotherapeutic molecule bound to a monoclonal antibody against a tumor cell surface protein, is approved by the FDA for the treatment of acute myelogenous leukemia. ACS Clinical Research Professor Irwin Bernstein, MD, and former grantee Eric Sievers, MD, developed the compound.
2000 After three years of study by ACS grantee Raymond Warrell, MD, arsenic trioxide (Trisenox) is approved as an orphan drug for treatment of patients with acute promyelocytic leukemia.
2000 The cox2 inhibitor, celecoxib (Celebrex) is approved by FDA for the prevention of colon cancer polyps in individuals carrying the APC gene for familial adenomatous polyposis, a form of colon cancer. Clinical trials for prevention of sporadic colon cancer and other cancers are ongoing.
2000 Former ACS grantee Donald Kufe, MD, reports that the first human clinical trials of endostatin prove that it is safe and may have anti-cancer potential.
2001 FDA approval of Gleevec (formerly STI 571) for treatment of chronic myelogenous leukemia, based on clinical trials conducted by Brian Druker, MD; initial tests of Gleevec in rare gastrointestinal tumor promising.
2001 Publication of the Human Genome Project sequence in the journal Science includes 43 (of 315 total) or 14% of the authorship as current and previously awarded ACS grantees.
2001 Former grantees Leonard Saltz, MD, and Albert LoBuglio, MD, report that IMC-C225 monoclonal antibody against the epidermal growth factor receptor plus the drug irinotecan, produce a response in colorectal cancers resistant to standard treatment in phase II trials.
2001 Former ACS Research Professor Leland Hartwell, PhD, won the Nobel Prize Prize in Physiology or Medicine. Dr. Hartwell was recognized for conceptually groundbreaking research on the cell division cycle, begun in the early 1970s with the help of an ACS grant.
2002 DNA microarray technology (Gene Chip) was successfully used by Stephen Friend, MD, PhD, to predict which node-negative breast cancer patients will go on to develop metastasis and thus benefit from aggressive adjuvant therapy.
2002 ACS Professor Bert Vogelstein, MD, announces a new screening test for colon cancer that detects specific genetic abnormalities in stool samples of up to 70% of patients with colon cancer.
2002 Using archived samples supplied by the Ovarian Genetic Clinic of former ACS Clinical
Oncology Fellow David A. Fishman, MD, scientists from the FDA and National Cancer Institute use the new science of proteomics combined with artificial intelligence to analyze patterns of blood proteins that were able to detect ovarian cancer at an early stage in women at high risk. Large scale trials for ovarian cancer detection begin in the fall nof 2002. Former grantee David Ornstein is studying the system for increasing the specificity of prostate cancer detection.
2002 Zevalin, a monoclonal antibody conjugated to a radioactive isotope, is approved by the FDA after patients with B-cell lymphoma achieve a 30% complete remission when treated with Zevalin plus Rituxan, compared to 16% with Rituxan alone. Zevalin trials were carried out by former ACS grantee Thomas Witzig, MD. Earlier Rituxan trials were under the directionof ACS Clinical Research Professor Ronald Levy, MD, who was the first todemonstrate that monoclonal antibodies were effective in the treatment of
lymphoma.
2002 Former grantee Mark Kris, MD, reports that gefitinib (Iressa), a small molecule inhibitor of the epidermal growth factor receptor had amazing success in patients with advanced non-small cell lung cancer that had failed cisplatin therapy. Ten per cent of patients had a 50% regression of their tumors, and 36% had improved symptoms.
2002 More good news about Gleevec–Brian Druker, MD, shows that 68% of CML patients taking Gleevec as front-line therapy had a complete remission, compared with 7% on the standard therapy of interferon. Only 4% of Gleevec patients had gotten worse, whereas 19% of interferon patients had progressive disease. Other investigators reported that Gleevec reduces tumors by half in 63% of people with rare, usually fatal gastrointestinal
stromal tumor.
2002 Michele Carbone, MD, presents evidence that mesothelioma may result from a synergy between asbestos exposure and infection with a monkey virus (SV40) that contaminated early polio vaccines. A combination of pemetrexed (Alimta) and cisplatin induces a partial response in 41% of mesothelioma patients compared to 17% with cisplatin alone; 70% of patients on the two-drug regimen had symptom improvement, according to
study director Nicholas Vogelzang, MD.
2002 FDA approves taxotere, developed by former ACS grantee David Kingston, PhD, as 1st line therapy for advanced lung cancer.
2003 Former grantee Gary Ruvkun, PhD, uses RNA interference (RNAi) to identify six genes leading to obesity in the roundworm. Technique is hailed by Nobel Laureate Phillip Sharp, PhD, as a “gift from heaven” that will allow researchers to discover all of the genes involved in cancer and other catastrophic diseases.
2003 Former grantees Michael Clarke, MD, and Max Wicha, MD, discover that the ability of breast cancer tumors to metastasize resides in just a few “breast cancer stem cells” that are highly resistant to chemotherapy.
2003 ACS In-house researchers, led by Eugenia Calle, PhD, conclude that overweight and obesity may contribute to most types of cancer and could account for 14 percent of cancer deaths in men and 20 percent of cancer deaths in women—an average of one out of every six cancers diagnosed.
2003 FDA approves Iressa as third-line therapy (after failing two conventional therapies) for non-small cell lung cancer.
2003 The FDA approves Velcade for use in the treatment of multiple myeloma, based on research and clinical trials conducted by former grantees Kenneth C. Anderson, MD, Sundar Jagannath, MD, Bart Barlogie, MD, and Paul Richardson, MD. Velcade, or bortezomib, is the first in a new class of drugs known as proteasome inhibitors. Also see 2004 entry below on Nobel Laureates Aaron Ciechanover, MD, DSc, Avram
2003 Building on the creative insight of former grantee Judah Folkman, MD, in the early1970s, Avastin (becacizumab) becomes the first antiagiogenesis drug to show success in large, randomized phase III clinical trials. Among the many investigators testing Avastin, an antibody directed against the vascular endothelial growth factor (VEGF) was former ACS grantee John D. Hainsworth, MD.
2003 The first chemoprevention trial for prostate cancer shows that the drug finasteride (trade name Proscar) reduces the risk of prostate cancer in men over 55 by 25%, although not without unwanted sexual side effects. In addition, a small percent of the tumors that did develop were more aggressive than in the placebo group. The trial was carried out by the Southwest Oncology Group, and included former ACS grantees Ian M.
Thompson, MD, Scott M. Lippman, MD, Charles A. Coltman, Jr., MD, and the late Gary J. Miller, MD, PhD.
2003 Annual Report to the Nation announces the death rates for lung, breast, prostate and colon cancers continue to decline and that incidence rates from 1995 through 2000 were stabilized.
2003 Former grantees Michael Snyder, PhD, Thomas Gingeras, PhD, and Anindya Dutta, PhD and colleagues embark on the Encyclopedia of DNA elements (ENCODE) project funded by National Human Genome Research Institute at $36 M to discover all parts of the human genome that are crucial to biological function for the purpose of realizing the full potential of the genome project to improve human health.
2004 Avastin (bevacizumab) becomes the first FDA approved antiangiogenesis drug. It was proven effective as a first-line treatment for metatstaic colon and rectal cancer in a randomized phase III clinical trial. This follows a 1997 announcement by former ACS grantees American Cancer Society Medal of Honor Awardee M. Judah Folkman, MD, and Timothy Browder, MD that their drugs angiostatin and endotstatin cured
cancer in mice by blocking the tumor’s blood supply. Former ACS grantee John D. Hainsworth, MD was involved in the testing of Avastin which is designed to inhibit Vascular Endothelial Growth Factor (VEGF), a protein that plays a critical role in the formation of new blood vessels to tumors.
2004 FDA approves Altima (pemetrexed disodium) for the treatment of patients with locally advanced or metastatic non-small cell lung cancer. The drug had previously been approved for the treatment of malignant mesothelioma, a type of cancer affecting the inside lining of the chest cavity when former grantee Nicholas Vogelzang, MD directed its clinical trial.
2004 Eugenia Calle, PhD, American Cancer Society director of the analytic epidemiology and colleagues find that U.S. overweight and obesity cause 90,000 cancer deaths per year, and impacts the nation in terms of both mortality and heathcare costs equaling or exceeding that associated with tobacco use.
2004 Grantees, Beth Jones, PhD, MPH, Hosanna Sola Via, PhD, and Christine Howe, PhD provide the first reported finding that African-American female breast cancer patients have a higher prevalence of p53 mutations than that in white women possibly contributing to the poorer prognosis for this disease found in blacks.
2004 Novel ABL kinase inhibitor, BMS-354825, complements Imatinib and successfully treats Gleevec – resistant chronic myelogenous leukemia as reported by former grantee, Charles Sawyers, MD.
2004 Current ACS Research Professor, Michael Karin, and colleagues provide the first evidence fo the molecular link between inflammation and cancer by determining that the enzyme I-kappa-B kinase beta (IKKB) is required for the activation of NF-kB which in turn activates a cascade of events that can lead to some types of cancer.
2004 — Annual Report to the Nation on the Status of Cancer, a collaborative report with the
American Cancer Society’s Surveillance Research Department, announce a 0.5% decrease in cancer incidence rates from 1991 to 2001, while death rates from all cancers combined dropped 1.1 percent per year from 1993 to 2001. The new data reflects progress in cancer prevention, early detection, and treatment and that not all segments of the U.S. population benefited equally from the advances.
2004 Aaron Ciechanover, MD, DSc, Avram Hershko, MD, PhD, and Irwin Rose, PhD jointly won the Nobel Prize in Chemistry for their roles in the discovery of ubiquitin-mediated protein degradation. Their work on this small protein called ubiquitin which tags unwanted proteins for proteosome destruction. These discoveries have led to the development of a new class of cancer-fighting drugs known as proteasome inhibitors. Also see 2003 entry above on Velcade.
2005 The National Cancer Institute (NCI) releases preliminary results from an ongoing clinical trial of bevacizumab (AvastinTM) for patients with previously untreated advanced non-squamous, non-small cell lung cancer after the study found patients who received the drug in combination with standard chemotherapy lived longer than patients who received the same chemotherapy without the drug. Former ACS grantee John D. Hainsworth,
MD was involved in the testing of Avastin; see 2004 entry above on Avastin.
2005 The first ACS national conference “Exploring Models to Eliminate Cancer Disparities Among African American and Latino Populations: research and Community Solutions” sponsored by the Behavoral Research Center convened designed to bring together researchers, healthcare providers, community groups, and advocates with the aim of discussing evidence-based strategies and initiatives to eliminate cancer
disparities.
2005 Genentech Phase III trials on the use of Herceptin plus chemotherapy to reduce recurrence of HER2+ breast cancer were stopped early due to significant improvement in overall survival. Results suggest for the first time that therapy which targets the genetic mutation has the potential to reduce disease recurrence. See 1988 entry on current ACS Clinical Research Professor Dennis Slamon, MD.
2005 Provenge becomes the first therapeutic vaccine for prostate cancer reaching phase III clinical trials status. One of the co-authors of the publications on its phase II trial was former grantee Frank Valone, MD.
2005 ACS supported grantees, Carla Wilson, MD and Bart Barlogie, MD begin a phase II-III trial of the thalidomide-like drug, lenalidomide (RevalidR) to treat multiple myeloma.
2005 Ahemdin Jemal, DVM, PhD and colleagues in the ACS Department of Epidemiology and Surveillance Research, reveal death rate trends from all cancers combined to first increase from 1970 to 1990 and then decrease through 2002, yielding a net decline of nearly 3%, while the death rate from all causes of death in the US combined decreased by 32 % between 1970 and 2002.
2005 American Cancer Society established the Statistics and Evaluation Center August 1, 2005 with the recruitment of Director, James L. Kepner, PhD, as a part of the Research and Training Program.
2005 Current grantee Peter Lee, MD provides evidence for counting the number of immune system dendritic cells and T cells in axillary lymph nodes rather than the number of cancer cells in sentinel lymph nodes as a method of predicting possible breast cancer recurrence – a prognostic profile indicator for clinical outcome.
2006 The first Special Initiative Professorships totaling $1,400,000 were awarded in 2006. Andrew Berchuck, MD was named to the ACS-Barbara Thomason Ovarian Cancer Professorship to identify genetic polymorphisms that affect ovarian cancer susceptibility, Maria Martinez, PhD was awarded the ACS-Richard H. Hollen Cancer Prevention Professorship to develop a program increasing colorectal screening in the state of Arizona, and Beth Karlan, MD was awarded the American Cancer Society California Division Early Detection Professorship to discover how to detect ovarian cancer
early, while the disease is more treatable than in the later stages. The Special Initiative Professorships awarded to Drs. Berchuck and Martinez are a result of donors providing generous gifts to fund specifically designated research on a particular type of cancer.
2006 A third Cancer Prevention Study called CPS-3, is launched by the Epidemiology and Surveillance Research Department to replace the aging population of CPS-II. The study will begin with pilot studies in Texas, Georgia, and California to test the feasibility of using the Relay for Life venue as a recruitment tool for long term surveying of 500,000 adult male and female participants.
2006 American Cancer Society celebrates the 60th anniversary of its Research Program with
outstanding scientific presentations of cancer research progress at the National Academy of Sciences in Washington, D.C where it first began 6 decades ago.
2006 For the first time the number of Americans dying from cancer has actually decreased slightly due to reduced number of lung cancer deaths in men during 2003.
2006 Former grantees Deborah Armstrong, MD and Joan Walker, MD provide evidence that chemotherapy delivered directly into the abdominal cavity coupled with surgery to remove advanced ovarian cancer tumors followed by standard intravenous chemothryerapy is superior to standard intravenous therapy after surgery alone. NCI encourages this use.
2006 Former grantee Matthew P. Scott, PhD finds sterol synthesis inhibitors, such as statins, reduce Sonic Hedgehog signaling and tumor proliferation in medulloblastoma.
2006 Preliminary results from the Study on Tamoxifen and Raloxifene (STAR) clinical trials reveal that raloxifene is just as effective as tamoxifen at reducing recurrence of invasive breast cancer. Both tamoxifen and raloxifene were developed by former grantee V. Craig Jordan, PhD, DSc.
2006 In June, the U.S. Food and Drug Administration (FDA) approved a new vaccine against the strains of the human papilloma virus (HPV) that cause most cases of cervical cancer. Former grantee Robert C. Rose, PhD discovered the usefulness of harmless viral particles as a method of producing the immune response in several cervical cancer vaccines developed.
2006 Two RFAs open to most investigators were launched: 1) Pilot and Exploratory Projects in Palliative Care of Cancer Patients and Their Familes, and 2) Role of Healthcare and Insurance in Improving Outcomes.
2006 TM-601, a new drug derived from scorpion venom by former grantee Harald Sontheimer, PhD is in phase I clinical trials for preventing tumor cell spread and metastais in brain cancer.
2006 Two Nobel Laureates: Craig C. Mello, PhD won the Nobel Prize in Physiology and Medicine along with Andrew Fire, PhD for their discovery of RNA interference (RNAi) – gene silencing by double-stranded RNA. RNAi is a small RNA involved in the flow of genetic information. See 1998 entry on RNAi. Former grantee Roger D. Kornberg, PhD was the sole winner of the Nobel Prize in Chemistry for his work on the molecular basis of eukaryotic transcription – how DNA is converted to RNA.
2006 FDA approves Avastin (Bevacizumab) for first-line treatment of patients with unresectable, locally advanced, recurrent or metastatic nonsquamous, non-small cell lung cancer. This anti-angiogenesis drug was originally approved as a first-line treatment for metatstaic colon and rectal cancer. Former ACS grantee John D. Hainsworth, MD was involved in the testing of Avastin which is designed to inhibit Vascular Endothelial Growth Factor (VEGF), a protein that plays a critical role in the formation of new
blood vessels to tumors.
2007 An American Cancer Society report, Cancer Statistics 2007, shows there was a drop of 3,014 cancer deaths in the United States from 2003 to 2004, the most recent year for which mortality data are available from the National Center for Health Statistics. This drop was significantly larger than the 369 fewer deaths reported for the previous time period (2002 to 2003), which itself marked the first decline in actual number of cancer deaths in the more than 70 years since nationwide data began to be compiled.
Decreasing numbers of cancer death in consecutive years suggests the rates will continue to decline.
2007 Overall five year relative survival rates reach 66% for cancers diagnosed between 1996 and 2002.
2007 American Cancer Society Breast Cancer Advisory Group modifies the Society’s Guidelines for Breast Screening to include an annual MRI exam for women at high risk for breast cancer greater than 20.
2008 An experimental urine test using four different biomarkers identified by Arul Chinnaiyan, MD, PhD, detects prostate cancer with an accuracy that outperforms conventional detection methods using a single marker. 2008 – An experimental urine test using four different biomarkers identified by Arul Chinnaiyan, MD, PhD, detects prostate cancer with an accuracy that outperforms conventional detection methods using a single marker.
2008 Melinda Sanders, MD, and Society Clinical Research Professor Carlos Arteaga, MD, find that a short preoperative treatment with erlotinib (Tarceva®), a drug approved for pancreatic cancer and non-small cell lung cancer, is effective in inhibiting growth in estrogen receptor – positive breast cancer.
2008 A study from the Women’s Health Initiative led by Electra Paskett, PhD, determines that women who smoke are almost twice as likely to develop rectal cancer as those who have never smoked.
2008 Society Clinical Research Professor Dennis Slamon, MD, PhD, identifies three overexpressed genes in a specific type of breast cancer responsive to the drug dasatinib, making it a potential treatment for breast cancers lacking common female hormone receptors.
My very uninformed opinion is that you have a charity that is started because there is a real need; it collects monies and most of it goes to the prime reason the charity started. Then the charity grows and so much money is spent on fundraising, that I’m wondering if all that much more money is going to the original cause; i.e. is there a point of diminishing returns? It’s a shame for so many donator dollars to go the administrative/fundraising part when those same dollars could be spent on another cause where the money is really going to the cause. I have had breast cancer twice and appreciated some of the service available but I am really done with the Pink Ribbon stuff. It is just so overdone that it is almost a turnoff. v
I am a breast cancer survivor – going on 13 years. I have learned that I am a direct beneficiary of American Cancer Society research funding – PAP smears, Tamoxifen, mammorgrams. I learned about the 46 Nobel prize winners for medical research funded by ACS by being involved – especially with the Relay for Life in my community. I believe, as others have noted, that research needs to be finding cures for as many types of cancers as possible, not just breast cancer. I would suggest that if one believes in the missions and causes of these organizations, he or she should get involved, even if it is only donating $5 or $10.
Comments are closed.